EndoPredict® Service
The only second-generation test that predicts the risk of recurrence for up to 15 years.

Citogen is the benchmark laboratory in Spain certified by the manufacturer Myriad for the performance of the EndoPredict (EP) prognostic and predictive breast cancer test. This test sets a risk score (EPclin Risk Score) using the 12-gene molecular expression profile along with the patient’s clinical data (affected lymph nodes and tumour size) to:
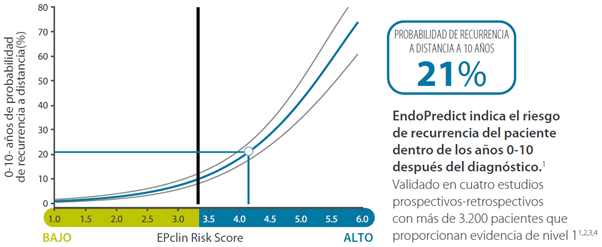
- Forecast 10-year recurrence risk (high/low risk)
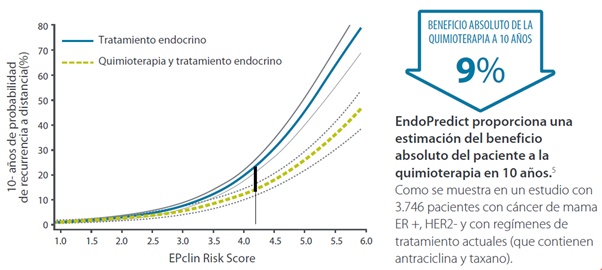
- Predict the absolute benefit provided by chemotherapy at 10 years
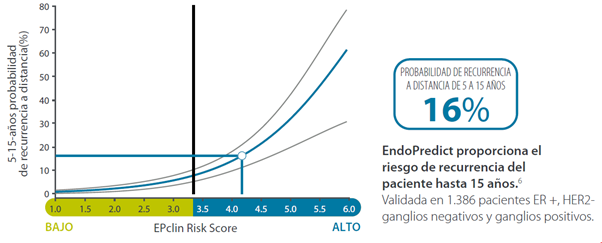
- Assist in decision-making about endocrine therapy as from 5 to 15 years

The test is intended for patients with early ER+, HER2-breast cancer with or without affected nodes. EP is used in patients who have not received systemic therapy (hormone or chemotherapy) or radiotherapy prior to sampling.
EndoPredict answers three important clinical questions
Can chemotherapy be avoided? - 10-year risk
What is the absolute benefit of chemotherapy at 10 years?
Can adjuvant endocrine therapy be stopped after 5 years? – Risk as from 5 to 15 years
Why request the EndoPredict test from Citogen?
- Report issued in as little as seven business days.
- Recommended by international guides such as ASCO, ESMO, St. Gallen, EGMT, AJCC, AUG or NCCN.
- Aids in therapeutic decision-making (8, 9).
- Accurate metastasis risk calculation for patients with negative or positive nodules.
- Clearly differentiated high and low-risk categories.
- Analytical performance and reproducibility.
- Prognostic and predictive capacity backed by multiple publications:
- Clinical validation in multiple prospective-retrospective studies with more over 3,500 ER+, HER2- patients (1, 2, 4, 7).
- Diagnostic performance validated by analysis of ER+, HER2- patients in phase III studies (ABCSG-6, ABCSG-8 and TransATAC), with consistent results (level 1B evidence) (1, 3, 6).
- Effect of EndoPredict on therapeutic decision-making, leading to a reduction in chemotherapy in between 10% and 33% of cases (9, 10).

Request Form
Sample Submission

Sample analysis

Report in seven business days
References:
- Filipits et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res. 2011.
- Martin M. et al., Clinical validation of the EndoPredict test in node-positive, chemotherapy-treated ER+/HER2- breast cancer patients: results from the GEICAM 9906 TRIAL. Breast Cancer Res. 2014.
- Buus R. et al. Comparison of EndoPredict and Epclin with Oncotype DX Recurrence Score for Prediction of Risk of Distant Recurrence After Endocrine Therapy. J Natl Cancer Inst. 2018.
- Sestak I. et al. Prediction of chemotherapy Benefit by EndoPredict in patients with breast cancer who received adjuvant endocrine therapy plus chemotherapy or endocrine therapy alone. Breast cancer Res Treat. 2019.
- Filipits et al. Prediction of Distant Recurrence using EndoPredict among Women with ER+, HER-Node-Negative Breast Cancer Treated with Endocrine Therapy only. Clin Cancer Res. 2019.
- Sestak I. et al. Comparison of the Performance of 6 Prognostic Signatures for Estrogen Receptor-Positive Breast Cancer A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol.2018.
- Constantinidou et al. Clinical Validation of EndoPredict in Pre-Menopausal Women with ER-Positive, HER2- Negative Primary Breast Cancer. Clin Cancer Res. 2022.
- Dinh et al. Impact of the EndoPredict genomic assay on treatment decisions for oestrogen receptor-positive early breast cancer patients: benefits of physician selective testing. Breast Cancer Res Treat. 2021
- Villareal-Garza et al. Change in therapeutic management after the EndoPredict assay in a prospective decision impact study of Mexican premenopausal breast cancer patients. PLoS One. 2020
- Penault-Llorca et al. Decision of adjuvant chemotherapy in intermediate risk luminal breast cancer patients: A prospective multicentre trial assessing the clinical and psychological impact of EndoPredict (EPclin) use (UCBG 2-14). Breast. 2020
To obtain advice on the EndoPredict assay, fill out the form and one of our technical experts will get in touch with you shortly